Physics 101
What we did
today!
Tues. Dec. 9 & Thurs. Dec. 11
Nanotechnology -- Wrap-up
Complete
multi-group discussions
Create study guide for final -- Link
Thurs. Dec. 4
Nanotechnology -- Health
& Ethics
Nanotechnology: Societal Implications & Health Issues
Discussion
Questions
Nano &
Health
1. In what places do we need to monitor health
risks of nanotechnology?
2. In what ways could nanomaterials enter our
bodies?
3. How do scientists/medical doctors study
toxicity to humans?
4. What other risks might there be in developing
nanotechnologies? (not personal health)
5. What does the equation, RISK
= TOXICITY X
EXPOSURE TIME, mean?
6. What precautions can be taken to minimize
risks?
Nano &
Society
1. What are the benefits to society from
Nanoscience and Nanotechnology & their Applications?
2. As you consider economic growth, what ethical
considerations need to be made as we move forward with
nanotechnological
development? How should we decide which
technologies to push most quickly?
3. In what areas in the past have new
technologies brought negative impacts as well?
Name them and the impact
4. How will branches of law be impacted by
nanotechnology?
5. What ethical issues might we come up against
as nanotechnology is implemented?
Risk – Benefit
Analysis
1. In a risk-benefit analysis, people must
decide which outweighs the other. List
benefits of nanotechnology and its applications on one side of the
paper and
risks or downsides of nanotechnology on the other.
RISKS
BENEFITS
2. Which wins in your mind? Is
there any way you might change your mind?
NIOSH and
NANO
Nanotoxicity: Ch. 13
Nanotechnology
DeMystified by
Linda Williams & Wade Adams
Nanotechhnology: Societal Implications
Tues. Dec. 2
Nanotechnology -- Au
Nanoshells & Medicine
Gold (Au) Nanoparticles and Nanoshells
How are gold nanoshells made?
- Silica cores
- Molecular coating to adhere Au particles
to core
- 1 nm Au particles added to molecules on
core
- Additional Au added and nanoparticle
heated until fully coated to thickness required <>
<>
<>How are Au nanoshells used?
- <>Additional molecular coating required to
"find" target cells in body
- Functionalized Au nanoshells sent into
body
- Au nanoshells find targeted cells and
congregate there
- Laser light helps image cells, or
- Laser light can heat up Au nanoshells to
destroy cells, or
- Ultrasound can heat up Au nanoshells to
destroy cells, or
- Heated Au nanoshells can deliver drugs to
targeted area
Medical
Applications of Nanoparticles and Nanomaterials
Nanoparticles used for Targeting
Cells and Assisting in Imaging Worrisome Parts of the Body
Au
Nanoshells target cancer
Au
nanoshells imaging abilities
Other medical applications that use Nano include:
Carbon
nanotubes used to detect lung
cancer by looking at organic chemicals in the breath
- A sensor capable of detecting and
recording which chemical species (among 200) was developed using CNTs
- Other diseases also are associated with
particular chemicals in the breath and may be able to be
studied/detected in this way in the near future.
Timed
Drug Delivery in Pills
- Using large encapsulated molecules and
then adding small reactant molceules
- Heating the capsule creates a reaction in
which long chains are formed which keep the drug from being delivered
in a single large dose
- Then Medicine is released over time
- < style="font-family: comic sans ms;">These
are already being made and advertised<><><>
<><>Tissue
Welding of Wounds
- <><>Nanoshells with albumin (protein)
"solder" coating are painted into cut edges of wound Near-infrared
lasers heat the nanoshells
- <><>The proteins denature and produce tissue
welding
Assisting
More Timely Bone Growth
- Short peptides self-assemble into a
collagen-like molecule
- Collagen then forms into long polymers
which build other peptide helices
- These collagen-like materials are
combined with nanocomposites for bone tissue engineering
Thurs. Nov. 25
Nanotechnology -- Carbon
Nanotubes
Carbon Nanotubes -- or CNTs
What are they?
- Carbon tubes of nanometer diameter formed from a flat sheet of
hexagonally oriented C atoms
- Different geometries/orientations of CNTs can form and each has a
different set of characteristics
- The longest CNT in the world to date was just reported at the
University of Cincinnati of 1 meter long
How
are they formed?
- CNTs are grown in one of two major ways
- In an arc furnace
- With a catalyst, like nanowires, via Vapor Liquid Solid growth
-- Co, Ni, or Fe used as catalysts (like Au nanospheres in nanowires)
General
Properties
- CNTs have:
- High conductivity (if metallic)
- High heat conductivity
- Are stronger than steel, yet flexible
- Are very light weight (as they are hollow)
- Armchair CNTs are mostly metallic
- Zig Zag and Chiral CNTs are only about 1/3 metallic and the rest
semiconducting
What
are they used for?
- Electrical components
- Sensors
- To strengthen materials
- As drug delivery systems
- See the links below
CNT Orientation In-Class Lab
Some Links:
IBM Nanotube
Applications -- Circuits
How Stuff
Works – nanotubes and more
More
NanoNews
General
CNT Info
Conductors, Semiconductors &
Insulators -- Energy bands instead of E levels -- Link
Tues. Nov. 18 and Thurs. Nov. 20
Nanotechnology -- Lectures
2 lectures on nanotechnology from Jan Y-R
Nanotechnology &
Nanowires: Here,There & Everywhere
How do we "look" at Nano Objects?
Thurs. Nov. 13
Nanotechnology -- Ch. 21 in Text Plus Provided Readings
What is
nanotechnology?
From Gary Stix article in Sept.
2001, Scientific American:
" After biomedical
research and defense-fighting cancer and
building missile shields
still take precedence-nanotechnology has become the most highly
energized discipline in
science and technology. The field is a vast grab bag of stuffthat has
to do with creating tiny
things that sometimes just happen to be useful. It borrows liberally
from condensed-matter
physics, engineering, molecular biology and large swaths of chemistry.
Researchers who once called themselves materials scientists or organic
chemists have transmuted
into nanotechnologists.
Purist academic types
might prefer to describe themselves as mesoscale engineers.
But it's "nano" that generates the buzz."
NANOTECHNOLOGY is a word
that is used to describe a plethera
of different
technological developments on a length scale of 100 nm or less (thus
the term nano).
It encompasses the fields of biology, chemistry, physics, and
engineering, as well as
medicine. We will focus on how physics and engineering have
changed -- what new
things can be done on a nanometer scale.
Invitation to
the Nano-World
Richard Feynman's 1959 lecture
on the idea of manipulating and
controlling
things on a small scale.
<http://www.zyvex.com/nanotech/feynman.html>
HOMEWORK
for TUESDAY, Nov. 18
Check out this special
article -- READ THE WHOLE
ARTICLE -- It
helps address these concerns.
<http://www.wtec.org/loyola/nano/IWGN.Public.Brochure/IWGN.Nanotechnology.Brochure.pdf>
This Special Edition has a bunch of
different Nano Articles: Link
Please read: Little Big Science,
Less is More in Medicine & glance through Tthe Incredible Shrinking
Circuit for Tuesday. Other articles in the journal
may also assist in your group's paper.
Also, Bring an article to class
for your group's topic. You may find one yourself or use
the resources above and below for your search.
TO assist with your papers: (I would open the links and choose which
sections/links to actually read/print depending on your paper focus
area)
Special Website
To Get
Things
Started -
Several articles are on this website --
latest in nanotechnology
Scientific American Article:
Oct. 2006, pp. 52-55
Taskforce
on Nanotechnology:
General overview, p.1-9
Good sections to read:
Theme 2: Future
Economic Scenarios, p.46-50
Theme 4: Future Social
Scenarios, p.54-59
Theme 7: Ethics, Governance,
Risk and Uncertainty, p.71-75
Feel free to pick other
sections.
The
National Nanotechnology Initiative:
News
articles from recent nanotechnological developments
Atoms: What are they?
What do they look
like? & What are their characteristics?
Looking at Atoms: Using the SEM, STM, and AFM
SEM: Scanning electron microscope
STM: Scanning tunneling microscope
AFM: Atomic force microscope
Click here for the PPT
link on the use of: SEM,
STM, and AFM
NOW.....
We have spectra -- how do we explain them?
At the turn of the century, we had the picture of an atom called
the
Raisin Pudding Model where the atom was the pudding with the electrons
being the raisins.
The result of Rutherford's experiment (done by graduate student,
Geiger, and undergraduate student, Marsden) was that the picture of the
atom had to be changed. He went back to Newton's Principia and
came up with the planetary model of the atom. This was then
modified by Bohr and others as we move toward the quantum mechanical
picture of the atom.
Structure of
the atom: nucleus with protons and neutrons & cloud of
electrons surrounding it
Atoms have an
energy level structure which is made up of the various allowed energies
an electron can have if it is excited by thermal means (heat) or
collisions
or by light.
The "orbit" we
talk about for electrons is a classical picture which does not take
into account
the quantum nature of atoms. The radius of the orbit is actually
the
most probable position of the electron, however the electron can be all
over
the place within the orbital cloud.

When
atoms absorb energy, they jump from a lower energy level to a higher
one. When atoms jump from a high energy level to a lower one,
they emit a photon of light whose energy is equal to the difference
between the upper and lower energies. In other words, light can
only be emitted or absorbed if
it is JUST ENOUGH to make the electron have the correct amount of
energy
for the new energy level.

How is light made?
Excitation of electrons to high energy levels is followed by electrons
dropping
to their ground (or un-excited states) This means they give up
energy to make the transition and that energy is
given off in the form of photons. Photons are packets of light
energy that have just the right amount of energy to
shift the electron from one energy level to the other.
Atoms of every element have a different number of
protons, neutrons, and electrons in it. You are
going to look at gases in this experiment. The electrons in the
gas tubes are excited by electrical current
flowing through them. When the electrons give up the extra energy
and go back to their original orbit
around the nucleus, they give off light of a certain color. Only
colors relating to the allowed energies
of the electron are given off. Each element has its own set of
allowed energies for its electrons and the
photons which are emitted form a SPECTRUM which is unique to
that atom. So, like a finger print,
we can identify what element is in the tube by recording what colors of
light are given off by the hot
gas.
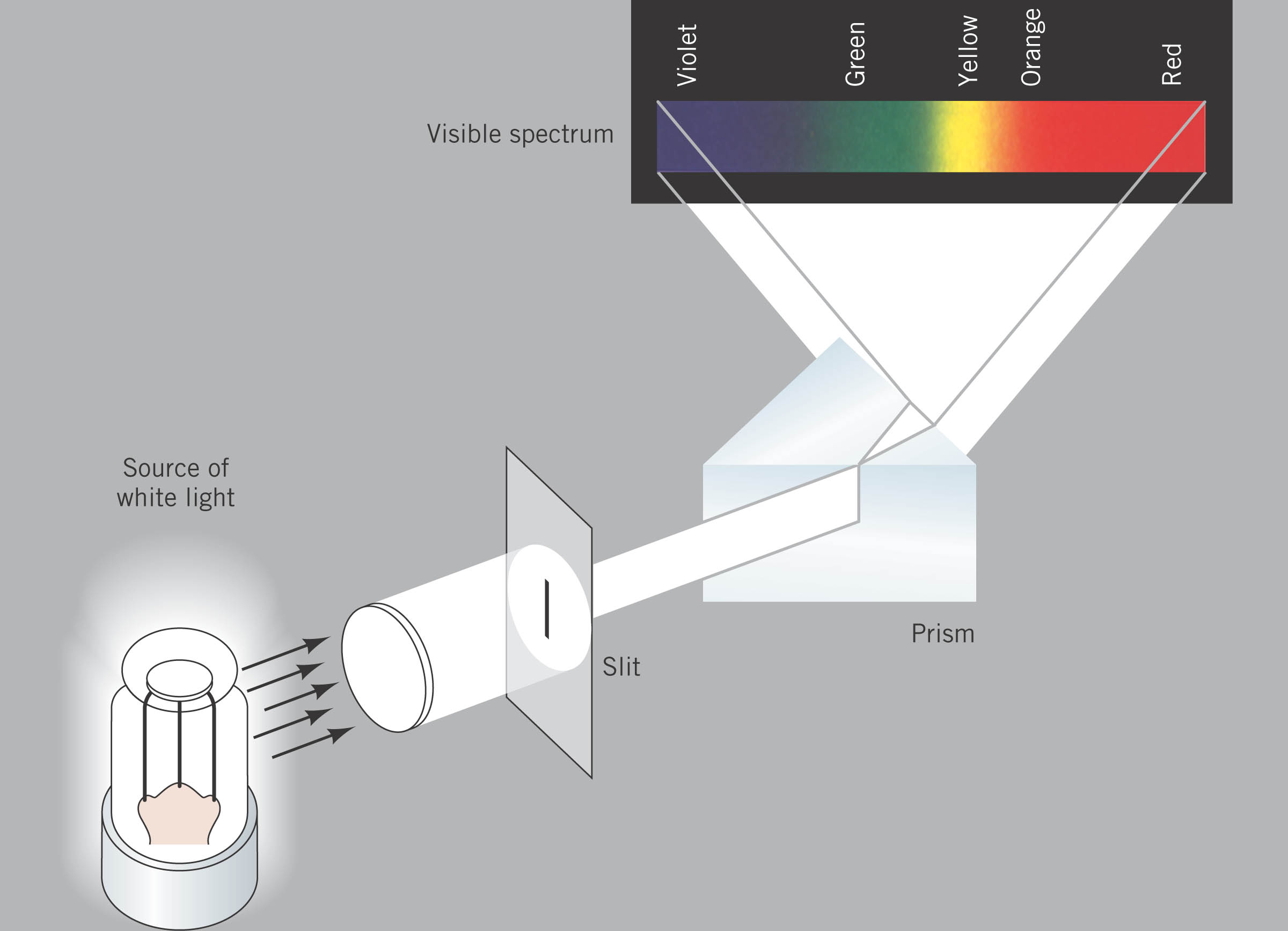
Light's Wave Nature:
Light propagates from one place to another as a wave. It has wave
properties of diffraction,
interference, reflection, and refraction.
Wave Properties:
Wavelength, lambda
Frequency, f
Period, T
T = 1/f
Wavelength * Frequency = Speed of Wave
Speed of light, c
Interference of Waves: Two waves superimpose on each other.
The amplitudes at a given location are added to find the
resulting waveform. Amplitudes
above the equilibrium point are positive and those below the
equilibrium
point are negative.
Constructive Interference: Occurs when both waves are in phase
with each other.
Destructive Interference: Occurs when each wave is out of phase
with the other, by 180 degrees.
Light's Particle Nature:
Planck was the physicist who derived the theoretical expression which
describes the intensity of light
given off as a function of wavelength for a given temperature black
body radiator. It was based on the
idea that each electron could only give off energy in discrete amounts
rather than any and all energies.
E = h f c =
l f = 3 x 108 m /s
In order to describe spectra, scientists had to model the atom with
different energy levels so that light
could be absorbed or emitted in discrete amounts also. The
photons which interact with the atoms have
to have just the right amount of energy to get the electron to jump
from one orbital to another (or one
energy level to another) or the photons are not absorbed or
emitted. This idea came from Einstein
when he studied the photoelectric effect.
CH. 21 HW
Review 14,15,17,23
Qs 6,9-12,16
Ps 1,2
Thurs. Nov. 6 & Nov. 11
Nanotechnology -- Ch. 21, Ch. 24 & 25 in Text Plus Provided Readings
What is nanotechnology? nanoscience?
Light
Microscopes -- TWO magnifying glasses together !!
How large is large, How small is small -- Short Video Clip
The Next Big Thing
The Next Big
Thing (only smaller)
 College of Engineering
College of Engineering
 The University of Wisconsin
- Madison
The University of Wisconsin
- Madison
Nanocutting Exercise --
and handout
How we look at atoms and very small things -- video clip and notes
Ch. 21 -- How do atoms and light interact
Nano-Sugar Calculation
Tues. Oct. 21st & Thurs. Oct. 23
The Nucleus -- Ch. 26 & Green Science
Butler Rural Electric presents: Personal Energy
Calculator and ENergy Library
Student Presentations:
Group 1: Nuclear Power
Group 2: Wind Power - no handout?
Group 3: Presidential
Candidates Views on Energy
Group 6: Alternate
E Cars
Group 7: The
melting -- water level
information
Group 8:
Different State's Energy Policies
Group 9: What can we do to reduce CO2
Group 10: Geothermal Info
Group 13: Energy Use of
Transportation Modes
Group 17: What can you do at Miami?
Group 18: Compressed Natural Gas
Group 20: Present US
Energy Policy
Group 22: Global reduction of
warming
Group 23: Important Facts on
Fossil Fuels
Group 26: US vs. Other Developed
Nations
Group 27: Cost & Emissions of
Various Alternative E
Group 28: Weather Issues with
Warming
Group 29: Damage attributed to
Global Warming
Group 30: Hydrogen Fuel Cells
Group 31: CO2
Footprint of Ethanol
Group 32: Best
Transporation Choices
Group 34: CO2
Footprint of Ethanol
Group 35: Other problems
associated with Alternate E Sources
The
atom/nucleus: ~1895
- Becquerel discovers "radioactive" materials: namely uranium salts
- Gives Pierre and Marie Curie the job of isolating radioactive
materials
- They share the Nobel prize for discovery of radioactivity and
radium.
- Rutherford finds alpha and beta rays looking at "Becquerel rays"
- Villard finds gamma rays
Radioactive
Decay:
- Alpha particles are helium nuclei -- 2 protons and 2 neutrons
- Beta decay depends on an electron either
- being produced when a neutron turns into a proton and then an
electron must be spit out of the nucleus
- being absorbed, or captured, by a proton so that it turns into
a neutron
- Gamma decay is a photon with very high energy
- 100s of KeV or Mev in energy
- compared to xrays in the 10s to 100 KeV
- compared to visible light in the 0.5 - 2 eV
- 233Pa 91
has 91 protons, 142 neutrons, 91 electrons, and 233 nucleons.
Radioactive
Decay Equations:
- Number of nucleons is conserved
- Element may transform up or down the periodic table to a
different element depending on the number of protons remaining after
the decay
- Element may stay the same and just release energy from the
nucleus (gamma decay)
- Charge is conserved
Ch. 26 Homework
Review: 26,27,35
Problems: 1,2
Nuclear Power -
what's new and how
does it work?
Green Science -- Wind Power,
Solar Energy, ETC.
Thurs. Oct 16
Science, Statistics, & Policies for Alternate Energy
Basic Articles to
Use for your Papers
Other
Helpful Articles/Cites
Extra Credit for Groups:
Tues. Oct 14
"Group Work on Papers"
The Grading Scheme and Basic "Outline" of
Paper Expectations for the:
- Individual Papers: 1 page not counting
Figures/Graphs/Pictures and REFERENCE
- Group Papers: 4 pages not counting Figures/Graphs/Pictures
and REFERENCES
Worked in groups to share articles on topics of choice and then decide
on jobs for each person in group.
Thurs. Oct 9
"Global warming: science & solutions" with Erick Avari
The latest scientific research
into alternate energy
Global
Warming Science & Solutions DVD
1. List each of the four major types of
change
that will occur due to the Greenhouse Effect, and add an example for
two of
them.
2. Describe two of the most interesting new
ideas for alternate energy sources that are being researched here in
the U.S.
as mitigating strategies for our fossil fuel-based economy.
3. What do the film makers suggest is the most
important thing for communities to do “right now” in terms of
adaptation?
4.
What final
thoughts does the narrator leave you with?
Basic Articles to Use for your Papers
Other
Helpful Articles/Cites
Thurs. Oct. 2
& Tues. Oct. 7
Al Gore's "An Inconvenient Truth" DVD
An Inconvenient Truth: A
Global Warning
!
With Al Gore and www.ClimateCrisis.net
As you watch the movie, Keep track of the following:
1. Important physical/scientific facts provided
2. Important policy issues discussed
3. When Al Gore politicizes the DVD, are the ill-timed comments
on science, or policy, or just as part of the "talk" he is giving
4. Was his personal life story affecting in terms of the DVD's
goal?
DAY 1 of the Movie Group Discussion
1. List 5 important
points that were presented on the DVD, and explain them in one sentence
2. What was the most surprising thing you
learned from this presentation?
DAY 2 of the Movie
Group Discussion Paper
Second time, same as before:
1. List 5 important
points that were presented on the DVD, and explain them in one sentence
2. What was the most surprising thing you
learned from this presentation?
Tues. Sept. 30th
Greenhouse
Effect & Alternate Energy
P
101: Energy Plan !
T.
Sept 30:
Exam return, form groups, Pick
Q
R. Oct 2:
Greenhouse
Effect Movie
T. Oct. 7
Greenhouse
Effect Movie, Pt 2
R. Oct. 9
Alternate
Energy Movie
T. Oct 14
Group
work – each person brings one article on topic
R. Oct. 16
Scientific
American Article on Alternate E
T.
Oct 21
Group Presentation
R. Oct. 23
Group
Presentations – Reports DUE
T. Oct 28
Thermal
Energy – Ch.
11
R. Oct. 30
Exam
#2
<> Groups:
1. Select your group (4 people per group)
2. Write down everyone's names and contact information
3. Fill out a Group Form to hand in
4. Choose a topic from list, or get an approved alternative.
5. Send a representative up to remove topic from list for others.
6. List your topic in Group Form
7. Decide on tasks for group members in preparation for working
on group paper.
8. Choose a group leader to be contact person with Jan Y-R and
provide info necessary for that
9. Hand in your Group Form for a Group #
Greenhouse Effect & Alternate
Energy Topics
Describe a
particular alternate energy – how does
it work, what is its cost, how long until it is ready for small scale
use –
large scale use
1. Wind power -
Group 2 - Brian Schlabig , Group Leader
2. Solar power
- Group 5 - Mohn Meyers
3. Nuclear
power - Group 1 - Mike Spiece
4. Compressed
natural gas - Group 18 - Matt Chacey
5. Any new
sources? - Group 10 - Matt Imielski
** New Topic: Hydrogen Fuel Cells - Group 30 - Scott
Stafford
6.
What
impact would Alt.E have on the world? - Group 4 - Mary
DelGrande
7.
What
will it take to reduce Greenhouse Effect globally?
8.
What can
I do (an individual family)? How much
would it cost/save me?
9.
What can
you do at Miami? How much would it cost/save me? - Group 17 -
Alex Josephs
10. What
are the arguments for local versus global adoption of Alt. E?
11. What
about the cooling effect of sulfur on the ocean? - Group 12 - Cody
Reichard
12. What
other problems do Alt. E. introduce (nuclear for example)? - Group 35
- Joe Montini (space for more)
13. What
social issues surround issues relating to Alt E and Greenhouse Effect?
(social,
population, ethical, economic status, etc) - Group 19 - Alex Schmidt
14. What
solutions are fast? Which may be feasible
in the future and why? At what cost? - Group 24 - Alex Kramer
As regards to
transportation:
15. What is
the best Alt E car available? - Group 6 - Adam Stagge
16. What
Alt E cars are being researched right now? - Group 14 - Charlie
Scheller
17. What
are the best vehicle/transportation sources? - Group 32 - Rebecca
Ferrenberg
18. How do
different modes of transportation utilize energy? Trains, planes,
Autos, etc - Gropu 13 - Kara Burghardt
19. What is
the CO2 footprint of ethanol and other possible fuel sources? - Group
31 - Christopher Napier
20. What
infrastructure would be required to convert to Alt E in the US? In
the
world? - Group 27 - Jodie Quinter
21. How do
the cost and the emissions of various alternatives compare? -
Economically/Governmentally:
22. What
effect does the Greenhouse effect have on the global economy? -
Group 25 - Max Bruno
** New Topic:
Greenhouse Effect on local economy - Group 34 - Sara Wenger
23. How do
Developed nations, Developing nations, and Underdeveloped nations look
at these
issues? – you can choose a single type of nation to report on, or
compare and
contrast - Group 16 - Andrew Settle
24. What
government regulation/energy policy exists presently in the US?
- Group 20 - Scott Stafford
25. How
does this compare to other developed nations? - Group 26 - Edward Rossi
26. What
energy policy do different states have that might be different than the
US
as a whole? - Group 8 - Allison Woodworth
27. What
are the Presidential candidates views on energy policy? - Group 3
- Emily Homel
28. What
about carbon credits and trading? How does it work? Is it viable for
the long
term?
As to the
Greenhouse Effect more specifically:
29. What do
we know about the iceberg melting, poles, coastlines? And what are the
predictions with various scenerios? - Group 7 - Jenna Seger
30. What
weather issues are there with the greenhouse effect with regard to
different
parts of the world? - Group 28 - Kaitlyn Talbot
31. What
health issues will be changing and coming up? - Group 21 - Heather
Baumer
32. How are
animals affected? - Group 15 - Samantha Ludington
33. How
much fossil fuel and other natural resources remain available in the
world? In
the US?
- Group 23 - Mike Russart
34. What
are natural sources of global warming and their results? - Group 11
- Molly Quin McMillan
35. What
type of damage has been attributed to global warming so far? With how
much
certainty? - Group 29 - Ali Bromberg
36. How
permanent is global warming?
37. On a
global scale, what will it take to reduce global warming? - Group 22
- Lauren Lubeck
** New
Topic - Individual person's Carbon footprint - Group 9 -
Sasha Young
EXAM #1
Thurs. Sept 18
& Tues. sept 23
Ch. 8 -- Energy
Main Ideas:
The unit of energy is the Joule, J, in the metric system.
A J = (k2 m2) / s2.
Interestingly, this is also the unit of Work.
Kinetic Energy
KE = 1/2 m v2
Is energy due to motion of an object. Any object in motion
will
exhibit KE. It is a
scalar quantity NOT a vector quantity.
Potential Energy
PE = m g h
Is energy due to position above or below a reference height.
PE
can be negative if
work must be done on the object to move it to your reference height.
Conservation of Energy
states that energy can neither be created nor
destroyed, it
simply changes states (or types of energy).
Conservation of Mechanical Energy states that a system that is
isolated can exhibit
conservation of mechanical energy. That is the total energy
of the system comes from the sum of PE and KE and as the system
evolves:
<>
KE + PE = Constant.
Examples of Conservation of Energy:
(1) A 1 kg pendulum swings from a starting position of
50
cm above equilibrium. What is its speed at the bottom of its
swing?
(2) The same pendulum swings to 15 cm above equilibrium.
What is its speed there?
Mini Experiment
Bouncing
Balls & Roller
Coaster
Experiments
1.
Bouncing Ball:
Ball, meter stick
a.
Choose a height to start from. hi =
b.
Drop ball.
c.
Record height ball returns to. hf =
d.
Calculate the following quantities: PE before, PE after, Speed ball hits ground
with.
e.
Is mechanical energy conserved
during fall? After return bounce? What happens to the energy of the ball?
f.
Choose another height to start from. Then calculate how fast the ball is moving
part of the way through the fall (for instance, drop ball from 75 cm
and see
how fast it is moving at 30 cm)
2.
Roller Coaster Problem: Loop-de-loop, car or ball, meter stick
<>a.
Make necessary measurements to
calculate the
following:
PE at top, Speed of car/ball at bottom, Speed of
car/ball at
top of loop
CH. 8 Homework
Qs 4,5,6,9,11
Ps 13,16
Tues. Sept. 9 &
Tues. Sept. 16
Ch. 2 & 3: Intro to
Physics & Society
Graphing:
Key Concepts:
Distance vs. Time Graph
Velocity vs. Time Graph
Acceleration vs. Time Graph
Reading Graphs & Creating Graphs
What does each type of graph look like for the following
situations:
Constant Motion
-- the distance increases with time (straight line with some slope, the
steepness of the line is related to the speed), the speed stays
the same regardless of time (straight horizontal line), the
acceleration is zero
Acceleration --
The distance
increases or decreases with time but the slope changes (as you speed up
the
slope increases, as you slow down the slope of the curve decreases)
thus
you see a curved line on distance graph, the speed incresases of
decreases with time in a linear fashion (straight line with some slope
corresponding to acceleration), the acceleration is a constant at all
times (straight horizontal line (non-zero, may be positive or
negative))
No Motion -- the
distance remains the same regardles of time (straight horizontal
liine), all other graphs are at zero (no speed and acceleration)
Changing Acceleration
-- all graphs are more complicated, distance changes with time
in a curved fashion and so does speed, accelearation is changing with
time (straight line at some angle)
Simple
Motion:
Mini-experiment In Class
Equipment: Meter sticks & stop watches
Using the above tools and the people in your group. Measure
the
following quantities.
a) Average speed of a person walking at constant pace.
b) Average acceleration of person starting from stop to a run.
c) Your response time when dropping a ruler between fingers
Experimental Procedure: Describe below how you would do
the
above 3 measurements.
How many trials would you do? Why?
Data: Record data below and show calculations of speed,
acceleration, and response time.
Analysis: Are the values you calculated reasonable? why
or why
not?
What are possible sources of error?
Looking back would you change your experiment in any way?
How?
HOMEWORK
ASSIGNMENT
Ch. 1
Qs. 5,6
Ps. 3c,d, 4c,d
Ch. 2
Qs. 1, 3-5, 7, 9
Ps. 4, 5
Ch. 3
Qs 2,3,9,
10 (graph), 13, 14 (graph), 15, 16
Ps
2, 15, 17-19 (graph), 21, 22
Thurs.SEpt. 4th
Investigating Aritcles -- WHat to
look for
Other ideas to consider when
reading critically:
1 Detecting
bias
2. Detecting misuse of numbers
?
?
?
How graphs
Add to your Understanding of the Article:
Scientific American
Article, Sept.
2006 Issue: "A Plan to Keep Carbon in Check."
Scientific American
Article, Sept.
2006 Issue: "The Nuclear Option."
Thurs.Aug. 28, 2008 -
Tues. Sept. 2
Science -- an intro
-- see websites listed below
Discussion about Physics
& Technology & Us
- Define physics
- What is technology?
- What is the difference between the two?
- How does society (YOU) interact with science/physics and
technology?
- WHat power/influence does society have on science and technology?
<>
Discussion
about Critical
Thinking
- What is critical thinking?
- How do YOU form an opinion?
- What is an informed opinion?
- How does one effectively argue a point?
Reading
Critically
- What are the facts
of the case?
- What are the
issues brought out in the study?
- Who is affected by
the problem (may or may not be persons directly mentioned in stories)?
- What are possible
directions one could take from where the story left off?
- What would be the
possible consequences of such actions?
- Ethical, social,
economic, and political implications
- Practical
constraints
Small
Group Discussion
Please
meet in your groups and answer the following questions.
1.
Describe the Greenhouse effect
2. What are the most important issues surrounding it?
3. What questions do you have about the Greenhouse effect?
4. Describe Alternate energy
5. What issues are there about it?
6. What questions about alternate energy do you have?
7. Describe Nanotechnology
8. What important issues surround nanotechnology?
9. What questions do you have about it?
READING
ASSIGNMENT:
Assignment #1:
Check
out your favorite newspaper online, or magazines like Scientific
American, Science, Omni, Stereo Review, National Geographic, etc.
Identify articles that have science /physics-related topics that affect
society. Then analyze it using
your critical thinking skills (under reading critically above) -- use
bullet points to answer the critical analysis questions. Bring
to class Thursday
Then, Read CH.
1 & 2 in Textbook



