| Note:
Performing your original search, what is the function of p protein in phloem translocation?, in PubMed Central will retrieve
15 citations. |
| Plant Cell. 2001 May; 13(5): 989–992. | PMCID: PMC1464708 |
Copyright © 2001, American Society of Plant Physiologists A Calcium-Regulated Gatekeeper in Phloem Sieve Tubes Nancy A. Eckardt, News and Reviews Editor |
The
phloem is a highly specialized long distance transport tissue in
vascular plants. Despite detailed knowledge of the development and
ultrastructure of phloem tissue in a wide variety of plant species,
surprisingly little is known about the mechanisms and regulation of
phloem transport. The main function of the phloem is transport of the
immediate products of photosynthesis (e.g., sugars) from “source”
tissue (actively photosynthesizing leaves) to “sink” tissue (immature
leaves, growing root tips, and developing flowers, fruit, and seed). It
is sometimes overlooked that a wide variety of other material is also
transported through the phloem, including proteins, amino acids,
solutes, viruses, and various signaling molecules. |
PHLOEM STRUCTURE AND FUNCTION All
students of plant physiology are familiar with the mass flow concept of
phloem transport. According to this concept, the transport of materials
through phloem sieve tubes is passive, nonselective, and driven
entirely by pressure gradients that are maintained by active loading of
photosynthate in source tissue and unloading of materials in sink
tissue. Phloem
tissue is well designed for long distance transport. The flow of
materials takes place through sieve tubes, which are made up of long,
tube-like, enucleate sieve elements arranged end to end and connected
by specialized end walls known as sieve plates. The sieve plates
contain large (up to 1 to 2 μm) pores that allow for passage of
materials between sieve elements. Typically, each sieve element in
angiosperms is accompanied by one or more companion cells, which
interact intimately with the sieve element and play a crucial role in
regulating phloem loading and unloading and in the turnover of sieve
element proteins and other components (reviewed by Oparka and Turgeon, 1999).
Sieve elements and companion cells are derived from unequal
longitudinal division of a single “fusiform mother cell.” The cell that
becomes the sieve element undergoes a highly regulated partial
autolysis, not unlike that of programmed cell death, in which the
central vacuole breaks down and the nucleus, ribosomes, cytoskeleton,
and Golgi bodies are degraded. The result is a large, nearly empty cell
that is well suited for conductance of a wide range of molecules.
However, specific components and organelles are retained that may have
functions in transport and/or cell–cell interactions within the sieve
tubes ( van Bel and Knoblauch, 2000). |
P-PROTEINS AND PHLOEM TRANSPORT Mature
sieve elements retain a modified endoplasmic reticulum (ER),
mitochondria, numerous types of proteins, and specialized sieve element
plastids. Most of these components are arranged along the lateral walls
of the sieve element and collectively constitute a system known as the
parietal layer ( Figure 1). A wide variety of sieve element proteins and plastids have been observed that can be distinguished by genus or family. 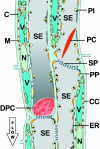 | Figure 1. Schematic Representation of the Histology of the Sieve Element/Companion Cell-Complex in Vicia faba. |
Behnke (1991a)( 1991b)
presented electron micrographs and descriptions of plastids and
crystalloid proteins from the sieve elements of many different species.
Behnke (1991a)
distinguished two types of sieve element plastids: S-type plastids,
which contain only starch inclusions, and P-type plastids, which
contain mainly protein inclusions. Behnke (1991b)
referred to “nondispersive” versus “dispersive” protein bodies, also
called P-proteins or structural sieve element proteins. These terms
arose because of changes that are observed during sieve element
ontogeny. Early in the maturation process, many protein bodies are
often observed, some of which disperse as the sieve element matures and
some that remain unchanged in mature sieve elements. There
are many shapes of nondispersive fibrous and crystalloid protein
bodies, also called P-proteins, which may be quite large and are often
observed in the lumen of sieve elements ( Figure 1).
Electron micrographic images sometimes show masses of fibrous or
amorphous protein located directly in front of or within the pores of
sieve plates and that appear to block transport through the pores. To
date, the large crystalloid P-protein bodies that occur exclusively in
Fabaceae were classified as being nondispersive ( Behnke, 1991b). It
has long been thought that some sieve element proteins may function to
block the pores of injured sieve tubes to prevent the loss of
assimilate. Sieve elements appear to be extremely sensitive to injury,
making it difficult to isolate and fix tissues for observation without
wounding them, possibly leading to an overabundance of micrographic
images with blocked pores. Some
researchers have considered the possibility that sieve element proteins
might regularly block mass flow through sieve tubes; thus, an
additional transport mechanism might be required to assist materials
through sieve tube pores. One possibility put forth was electro-osmosis
(see Spanner, 1979)
involving either a potassium ion or a proton pump connected to the
sieve plate to maintain sufficient pressure to allow the flow of
assimilates across the plate. Other theories invoked an active role for
sieve element proteins. Evert (1982)
reviewed various theories for phloem transport and concluded that the
pressure flow mechanism remained the most likely; he also stated that
it was difficult to conceive an active role for sieve element proteins,
or P-proteins, in phloem transport. For one thing, the amount and
structure of sieve element proteins are highly variable among different
plant families. Many monocots and some dicots appear to lack
crystalloid P-proteins entirely. Also, it has been shown that some
“nondispersive” crystalloid P-protein can disperse and move very
rapidly to plug sieve pores after injury. Evert concluded that the most
likely function of P-protein is to seal the sieve plate pores of
injured sieve elements as a rapid first line of defense against the
loss of assimilates. It is
possible to obtain electron micrographs from well-preserved and largely
uninjured sieve tubes using gentle tissue preparation techniques. Evert (1982) showed an electron micrograph of Cucurbita maxima sieve tubes with completely unoccluded pores. Ehlers et al. (2000) presented a detailed analysis of sieve elements in Vicia faba (broad bean) and Lycopersicon esculentum
(tomato). Well-preserved sieve elements clearly showed open, unoccluded
sieve pores. Deposits of stacked ER cisternae, plastids, and proteins
were observed along the parietal walls and along the walls of sieve
plates (particularly in V. faba), but gaps that were free of
materials were maintained in front of the sieve pores. These authors
also showed that, after injury, the sieve plate pores became occluded
by dispersed P-proteins, but the sieve element organelles remained
intact and in place along the parietal walls. Numerous clamp-like
structures were observed that appeared to anchor the plastids,
mitochondria, and ER cisternae in place along the plasma membrane of
the parietal wall. The clamps appear to be specific to organelles of
the sieve elements and likely function to prevent the components of
sieve elements from being carried along through the sieve tubes by the
mass flow turbulence. |
P-PROTEIN IS AN ACTIVE PARTICIPANT IN THE REGULATION OF PHLOEM TRANSPORT Knoblauch and van Bel (1998) used confocal laser scanning microscopy to visualize fluorescent dyes moving in sieve tubes of V. faba, which provided definitive evidence of unimpeded mass flow in intact plants. This study also reported that P-type plastids in V. faba
actually exploded upon injury of sieve elements, releasing their
protein contents, which, together with the dispersed crystalloid
protein, rapidly occluded the sieve plate pores. In this issue of The Plant Cell, Knoblauch et al. (pages 1221–1230) extend their previous work with confocal microscopy to show that crystalloid P-proteins of V. faba
rapidly disperse and occlude sieve plate pores after injury or osmotic
shock. Furthermore, they make the striking observation that this
process is rapidly reversible and controlled by calcium fluxes. It is
shown that protein from the large crystalloid protein bodies in V. faba
sieve elements dispersed to plug the sieve plate pores after injury
from micropipette injection or osmotic shock induced by various
osmolytes. Addition of the chelating agent EDTA completely prevented
crystalloid P-protein dispersal, and repeated exchanges of Ca 2+-
and EDTA-containing media induced the alternate dispersal and
re-assembly, respectively, of crystalloid P-proteins in injured sieve
tubes. This work represents an important advance in our knowledge of
phloem transport. Data from the current study, together with that of Knoblauch and van Bel (1998), suggest that P-protein originating from all sources within V. faba
sieve elements (e.g., P-plastids, parietal proteins, and larger
crystalloid P-proteins) takes part in the occlusion of sieve plate
pores after injury to cells. Repeated observations now suggest that
crystalloid proteins in V. faba are much more sensitive to
perturbation than are the P-type plastids. Unlike the reversibility of
crystalloid protein dispersal, explosion and dispersal of the P-type
plastids appears to be irreversible and presages the death of a sieve
element (M. Knoblauch and A.J.E. van Bel, personal communication).
Dispersal of parietal proteins after injury has also been observed and
was found to be more sensitive to perturbation than was the explosion
of P-type plastids ( Knoblauch and van Bel, 1998). In
addition to the “structural” sieve element proteins discussed here, a
wide variety of soluble proteins have been reported from phloem
exudates of many plant species. Hayashi et al. (2000)
reported that more than 100 polypeptides could be detected in phloem
exudates from a variety of species, including wheat and rice. Unlike
the protein composition of other plant tissues, low-molecular-weight
proteins appeared to be the dominant proteins in the phloem. In
addition, thioredoxin h, glutaredoxin, and glutathione
reductase have been found in reasonably high concentrations in phloem
exudates, suggesting a redox mechanism for the regulation of sieve tube
function. Interestingly, some of the principal sieve element proteins
from Oryza sativa, C. maxima, and Ricinus communis can interact with plasmodesmata to increase their size exclusion limit. Two C. maxima proteins, CmPP16 and CmPP36, also appear to mediate the transport of RNAs ( Xoconostle-Cázares et al., 1999, 2000).
It is likely that the primary function of many of these proteins is the
regulation of macromolecular trafficking via the unilaterally branched
plasmodesmata located at the junction of sieve elements and companion
cells ( van Bel and Knoblauch, 2000).
As shown by Knoblauch et al. in the present work, other phloem-specific
proteins appear to function in the regulation of conductance through
the sieve plate pores. |
A GENERAL PHENOMENON? Large
crystalloid P-proteins are a particular characteristic of the Fabaceae
(legumes). Knoblauch et al. examined sieve elements of Urtica dioica (Urticaceae) and Rubus fruticosus
(Rosaceae) in response to wounding and found that the P-protein bodies
present in these species failed to disperse and occlude sieve pores,
even when severely damaged in the presence of free Ca 2+.
P-type plastids also appear to have somewhat limited distribution.
Proteinaceous P-type plastids were observed in just 64 of 382 dicot
families studied by Behnke (1991a); the majority of families contained starch-filled S-type plastids. Calcium
is an important component of many signal transduction pathways, and
calcium regulation has been implicated in phloem function. In the
current study, Knoblauch et al. show that an influx of calcium into
legume sieve elements stimulates the rapid and reversible dispersal of
crystalloid P-protein to occlude sieve plate pores. It will be
important to determine if this phenomenon is limited strictly to the
Fabaceae or if other families have similar mechanisms, perhaps
involving the parietal and/or other P-proteins or plastids. The
concentration of free calcium in sieve tubes of R. communis has been found to be significantly higher than that in surrounding tissue ( Brauer et al., 1998), and calcium-dependent protein kinases have been detected in rice phloem sap ( Nakamura et al., 1993). Volk and Franceschi (2000) showed evidence of a calcium channel in the sieve element plasma membrane of tobacco and of the aquatic plant Pistia stratiotes
using immunolabeling with antibodies to a calcium channel protein.
These authors proposed that calcium channels become activated during
wounding or pathogen attack, facilitating calcium influx into phloem
tissues. McEuen et al. (1981) detected a calcium binding protein distinct from calmodulin in phloem exudates of C. maxima
and speculated that it might be associated with P-protein function. Do
any of these species have a calcium-regulated reversible mechanism for
sealing sieve elements? The grasses may represent an interesting case
for the investigation of sieve tube sealing after injury; they appear
to lack structural sieve element proteins (i.e., crystalloid or fibrous
P-proteins and parietal proteins), and sealing of injured sieve
elements might be achieved only by the contents of exploded plastids
and/or the slower process of callose formation. |
References Behnke, H.D. (1991a). Distribution and evolution of forms and types of sieve-element plastids in the dicotyledons. Aliso 3: 167–182. Behnke,
H.D. (1991b). Nondispersive protein bodies in sieve elements: A survey
and review of their origin, distribution and taxonomic significance. IAWA Bull. 12: 143–175. Brauer,
M., Zhong, W.-J., Jelitto, T., Schobert, C., Sanders, D., and Komor, E.
(1998). Free calcium ion concentration in the sieve-tube sap of Ricinus communis L. seedlings. Planta 206: 103–107. Ehlers,
K., Knoblauch, M., and van Bel, A.J.E. (2000). Ultrastructural features
of well-preserved and injured sieve elements: Minute clamps keep the
phloem transport conduits free for mass flow. Protoplasma 214: 80–92. Evert, R. (1982). Sieve-tube structure in relation to function. BioScience 32: 789–795. Hayashi,
H., Fukuda, A., Suzui, N., and Fujimaki, S. (2000). Proteins in the
sieve element-companion cell complexes: Their detection, localization
and possible function. Aust. J. Plant Physiol. 27: 489–496. Knoblauch, M., and van Bel, A.J.E. (1998). Sieve tubes in action. Plant Cell 10: 35–50. Knoblauch,
M., Peters, W.S., Ehlers, K., and van Bel, A.J.E. (2001). Reversible
calcium-regulated stopcocks in legume sieve tubes. Plant Cell 13: 1221–1230. [PubMed]McEuen, A.R., Hart, J.W., and Sabnis, D.D. (1981). Calcium-binding protein in sieve tube exudate. Planta 151: 531–534. Nakamura, S., Hayashi, H., Mori, S., and Chino, M. (1993). Protein phosphorylation in the sieve tubes of rice plants. Plant Cell Physiol. 34: 927–933. Oparka, K.J., and Turgeon, R. (1999). Sieve elements and companion cells: Traffic control centers of the phloem. Plant Cell 11: 739–750. [PubMed]Spanner, D.C. (1979). The electroosmotic theory of phloem transport: A final restatement. Plant Cell Environ. 2: 107–121. van
Bel, A.J.E., and Knoblauch, M. (2000). Sieve element and companion
cell: The story of the comatose patient and the hyperactive nurse. Aust. J. Plant Physiol. 27: 477–487. Volk, G.M., and Franceschi, V.R. (2000). Localization of a calcium channel–like protein in the sieve element plasma membrane. Aust. J. Plant Physiol. 27: 779–786. Xoconostle-Cázares,
B., Xiang, Y., Ruiz-Medrano, R., Wang, H.L., Monzer, J., Yoo, B.-C.,
McFarland, K.C., Franceschi, V.R., and Lucas, W.J. (1999). Plant
paralog to viral movement protein that potentiates transport of mRNA
into the phloem. Science 283: 94–98. [PubMed]Xoconostle-Cázares,
B., Ruiz-Medrano, R., and Lucas, W.J. (2000). Proteolytic processing of
CmPP36, a protein from the cytochrome b5 reductase family, is required for entry into the phloem translocation pathway. Plant J. 24: 735–747. [PubMed]
|
| |



 PROTEINS AND PHLOEM TRANSPORT
PROTEINS AND PHLOEM TRANSPORT