The Nitrogen Cycle
Explained by: Brenton W. Thomas
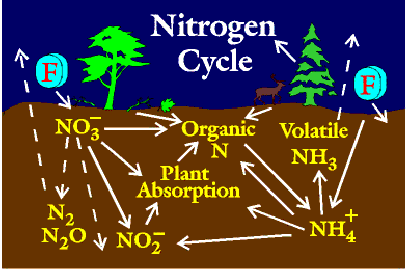
Overview: The nitrogen cycle involves three major steps: nitrogen fixation, nitrification, and denitrification. It is a cycle within the biosphere which involves the atmosphere, hydrosphere, and lithosphere. Nitrogen is found in several locations, or reservoirs. It is most prevalent in sediments and rocks, second in the atmosphere (78%).
Approximately 78% of air is Nitrogen. Nitrogen is important to life because it is a key part of amino and nucleic acids. Also, it is an important part of ATP, which is the basic energy molecule for living things.
Neither plants or animals can obtain nitrogen directly from the atmosphere. Instead, they depend on a process known as nitrogen fixation. Key players in this process are legumes and the symbiotic bacteria which are associated with the legume's root nodules. These bacteria are known as nitrogen-fixing bacteria. These organisms convert nitrogen in the soil to ammonia, which can then be taken up by plants. This process also occurs in aquatic ecosystems, where cyanobacteria participate.
After nitrogen has been fixed, other bacteria convert it into nitrate, in a process known as nitrification. In the first step of this process, Nitrosomonas convert ammonia into nitrite, and in the second step, nitrite is converted into nitrate, by Nitrobacter. This nitrate is then consumed by plants.
The final aspect of the nitrogen cycle is the process of denitrification. This process is performed by a variety of microscopic bacteria, fungi, and other organsims. Nitrates in the soil are broken down by these organsisms, and nitrogen is released into the atmosphere. This complete the cycle.
Nitrogen fixation is an anaerobic (without oxygen) process in which atmospheric nitrogen (N2)is reduced to NH3. Bacteria are responsible for this process. Bacteria in terrestrial and aquatic(water) environments participate in this process. These organisms must have a special enzyme known as dinitogenase to be able to to this.
Plants cannot use the nitrogen in our atmosphere without the assistance of nitrogen-fixing bacteria. These bacteria reduce atmosphereic nitogen to ammonia, which can be used to make other biological compounds. The plants do not use the ammonia directly, but it's a product of the waste.
Nitrogen is an essential plant nutrient. It is the nutrient that is most commonly deficient, contributing to reduced agricultural yields throughout the world. Molecular nitrogen or dinitrogen (N2) makes up four-fifths of the atmosphere but is metabolically unavailable directly to higher plants or animals. It is available to some species of microorganism through Biological Nitrogen Fixation (BNF) in which atmospheric nitrogen is converted to ammonia by the enzyme dinitrogenase. Microorganisms that fix nitrogen are called diazotrophs.
BNF requires energy. Those microbes that fix nitrogen independent of other organisms are called free living. The free-living diazotrophs require a chemical energy source if nonphotosynthetic, whereas the photosynthetic diazotrophs utilize light energy. The free-living diazotrophs contribute little fixed nitrogen to agricultural crops. Associative nitrogen-fixing microorganisms are those diazotrophs that live in close proximity to plant roots (that is, in the rhizosphere or within plants) and can obtain energy materials from the plants. They may make a modest contribution of fixed nitrogen to agriculture and forestry, but quantification of their potential has not been established. The symbiotic relationship between diazotrophs called rhizobia and legumes (for example, clover and soybean) can provide large amounts of nitrogen to the plant and can have a significant impact on agriculture.
The symbiosis between legumes and the nitrogen-fixing rhizobia occurs within nodules mainly on the root and in a few cases on the stem. A similar symbiosis occurs between a number of woody plant species and the diazotrophic actinomycete Frankia. The plant supplies energy materials to the diazotrophs, which in turn reduce atmospheric nitrogen to ammonia. This ammonia is transferred from the bacteria to the plant to meet the plant's nutritional nitrogen needs for the synthesis of proteins, enzymes, nucleic acids, chlorophyll, and so forth.
Plants receive the components of the "fixed" nitorgen using nitrates in the soil to provide the nutrients they need. Bacteria such as Nitrosomonas, Nitrococcus, and Nitrobacter participate.
Nitrification involves two steps. First, the ammonium ion (NH4+) is oxidized into NO2-. Then, this compound is further oxidized into NO3-. Again, bacteria in the soil participate in both processes.
Plant roots assimilate Nitrogen mainly in the form of nitrates while animals assimilate their nitrogen by eating the plants.
Ammonia is formed in the soil by the decompostion of plants and animals and by the release of animal waste.
This is the reduction of nitrates to gaseous nitrogen. Denitrifying bacteria perform almost the reverse of the nitorgen fixing bacteria.
1. List the three major processes involved in the nitrogen cycle.
2. Starting with nitrogen, draw a map showing the chemical transformations it undergoes throughout the cycle.
3. Describe the significance of legumes in the global nitrogen cycle.